Abstract
Background: Pelabresib (CPI-0610) is a potent, first-in-class, selective, oral small-molecule inhibitor of bromodomain and extraterminal domain (BET) proteins which is able to modify the expression of genes involved in nuclear factor kappa B (NFκB) signaling in patients with myelofibrosis (MF). Here we present results from MANIFEST (NCT02158858), an ongoing, global, open-label Phase 2 study investigating pelabresib monotherapy in patients with advanced MF who are intolerant/refractory to, or ineligible for ruxolitinib (RUX) and typically have very poor prognosis.
Methods: Eligibility criteria are MF patients intolerant/refractory to or ineligible for JAKi, Dynamic International Prognostic Scoring System (DIPSS) risk category of ≥intermediate-2, platelets ≥75 × 10 9/L, and ≥2 symptoms measurable (score ≥1) per Myelofibrosis Symptom Assessment Form (MFSAF) v4.0. Additional criteria include red blood cell (RBC) transfusion dependent (TD) per Gale criteria in TD cohort or spleen volume of ≥450 cc by computed tomography/magnetic resonance imaging in non-TD cohort. Patients were enrolled as TD (defined as ≥2 U RBCs/month over 12 wks) and non-TD if TD criteria are not met. The primary endpoint in TD cohort is RBC transfusion independence (TI; defined as no transfusion for ≥12 wks), and ≥35% spleen volume reduction (SVR35) at wk 24 in the non-TD cohort. Secondary endpoints include number of patients with ≥50% total symptom score reduction (TSS50) per MFSAF v4.0 at wk 24, and safety. Additional exploratory endpoints include changes in plasma levels of proinflammatory cytokines and bone marrow (BM) morphology/fibrosis. Patients with assessment at wk ≥24 and those discontinuing after wk 12 are included in the analysis of the corresponding endpoint; these were the evaluable patients.
Results: As of 29 September 2020, 27 pts were treated in the non-TD cohort for a median duration of 51 wks (2, 147 wks). At wk 24, 30% (7/23) evaluable pts achieved SVR35 (median change: -29%), and 48% (10/21) pts achieved TSS50 (median change: -56%). In the TD cohort, 19 pts were treated for a median duration of 32 wks (5, 78 wks). 21% (3/14) evaluable TD pts achieved RBC TI for ≥12 wks. Updated 24-wk data with a larger data set and new long-term data at 48 wks will be presented.
Pt subgroup analyses revealed evidence of activity of pelabresib in a subset of pts who were ineligible to receive RUX, a patient population that generally has few therapeutic options. Clinical benefits observed with pelabresib included achievement of SVR35 and TSS50, improvements in bone marrow fibrosis, and increases in hemoglobin levels.
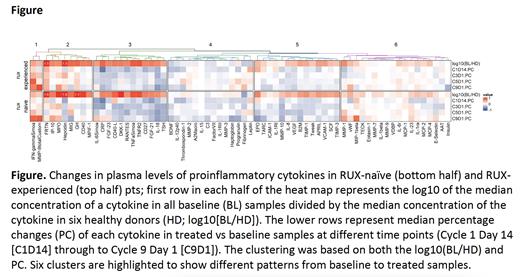
A panel of 68 cytokines, including those known to be nuclear factor kappa B (NF-κB) targets linked to inflammation and elevated in MF pts, were evaluated in plasma samples obtained at baseline (BL) and during therapy. Cytokines were clustered to show different patterns of change during treatment with pelabresib. Overall, pelabresib significantly reduced plasma levels of several cytokines in RUX naïve or experienced pts (Figure). Cytokine changes with pelabresib in cluster 3 (which includes IL-6, CRP, RANTES, TNFa and IL-18, and is characterized by higher BL values and bigger decreases over time) and in cluster 5 (which includes EPO, TARC, ICAM-1 and IL-8, and is characterized by relatively lower BL values and less profound decreases over time) were more pronounced in RUX-naïve pts.
46 pts were evaluable for safety. The most common hematological treatment emergent adverse events (TEAEs) of any grade were thrombocytopenia (30%; ≥Grade 3: 15%) and anemia (15%; ≥Grade 3: 13%). The most common (≥20%) nonhematological TEAEs were nausea (39%; no ≥Grade 3), diarrhea (37%; ≥Grade 3: 4%), dysgeusia and asthenic conditions (30% each; no ≥Grade 3 for either), respiratory tract infections (28%; ≥Grade 3: 2%), cough (26%; no ≥Grade 3) and constipation and weight decrease (22% each; ≥Grade 3: 2% each).
Conclusions: Preliminary data suggested pelabresib monotherapy was generally well tolerated and demonstrated signals of clinical activity in MF pts intolerant/refractory to or ineligible for JAKi, who have limited treatment options and poor outcomes.
Kremyanskaya: Astellas: Research Funding; Constellation: Research Funding; Incyte: Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Astex: Research Funding; Chimerix: Research Funding. Mascarenhas: Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Galecto: Consultancy; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Geron: Consultancy; Promedior: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forbius: Research Funding; CTI Biopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merus: Research Funding; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Research Funding; Prelude: Consultancy. Palandri: Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; AOP: Membership on an entity's Board of Directors or advisory committees; CTI: Consultancy. Vannucchi: Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Verstovsek: Celgene: Consultancy, Research Funding; NS Pharma: Research Funding; AstraZeneca: Research Funding; CTI BioPharma: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding; Ital Pharma: Research Funding; PharmaEssentia: Research Funding; Blueprint Medicines Corp: Research Funding; Sierra Oncology: Consultancy, Research Funding; Gilead: Research Funding; Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Harrison: Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sierra Oncology: Honoraria; Incyte Corporation: Speakers Bureau; Constellation Pharmaceuticals: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Bose: CTI BioPharma: Honoraria, Research Funding; Blueprint Medicines: Honoraria, Research Funding; NS Pharma: Research Funding; Astellas: Research Funding; Promedior: Research Funding; Pfizer: Research Funding; Constellation Pharmaceuticals: Research Funding; Sierra Oncology: Honoraria; Kartos Therapeutics: Honoraria, Research Funding; Novartis: Honoraria; Celgene Corporation: Honoraria, Research Funding; Incyte Corporation: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Schiller: Ono-UK: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Deciphera: Research Funding; FujiFilm: Research Funding; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sangamo: Research Funding; Actuate: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Geron: Research Funding; Genentech-Roche: Research Funding; Tolero: Research Funding; Takeda: Research Funding; Forma: Research Funding; Astellas: Honoraria, Research Funding, Speakers Bureau; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gamida Cell Ltd.: Research Funding; Arog: Research Funding; Karyopharm: Research Funding; Onconova: Research Funding; Celator: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; PrECOG: Research Funding; Regimmune: Research Funding; Mateon: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Samus: Research Funding; Bio: Research Funding; Delta-Fly: Research Funding; Trovagene: Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Elevate: Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Sanofi: Honoraria, Research Funding, Speakers Bureau; Pharma: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Biomed Valley Discoveries: Research Funding; Eli Lilly: Research Funding; ASH foundation: Other: Chair-unpaid; Sellas: Research Funding; Ono: Consultancy; Incyte: Consultancy; Ariad: Research Funding; AstraZeneca: Consultancy; Kaiser Permanente: Consultancy; Cyclacel: Research Funding; MedImmune: Research Funding; Ambit: Research Funding; Leukemia & Lymphoma Society: Research Funding; Bluebird Bio: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Rampal: Jazz Pharmaceuticals: Consultancy; BMS/Celgene: Consultancy; Stemline: Consultancy, Research Funding; Sierra Oncology: Consultancy; Novartis: Consultancy; Pharmaessentia: Consultancy; CTI: Consultancy; Abbvie: Consultancy; Blueprint: Consultancy; Disc Medicine: Consultancy; Memorial Sloan Kettering: Current Employment; Incyte: Consultancy, Research Funding; Kartos: Consultancy; Constellation: Research Funding. Drummond: BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gupta: Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy; Constellation Pharma: Consultancy, Honoraria; Roche: Consultancy; Incyte: Honoraria, Research Funding; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Patriarca: Novartis: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Argenix: Honoraria. Scandura: Constellation: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MPN-RF (Foundation): Research Funding; CR&T (Foudation): Research Funding; European Leukemia net: Honoraria, Other: travel fees . Teichmann: Pfizer: Membership on an entity's Board of Directors or advisory committees. Hoffman: Novartis: Other: Data Safety Monitoring Board, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; AbbVie Inc.: Other: Data Safety Monitoring Board, Research Funding; Kartos Therapeutics, Inc.: Research Funding. Colak: Constellation Pharmaceuticals: Current Employment. Ren: Constellation Pharmaceuticals: Current Employment. Bobba: Constellation Pharmaceuticals: Current Employment. Cui: Constellation Pharmaceuticals: Current Employment. Efuni: Constellation Pharmaceuticals: Current Employment. Talpaz: Imago: Consultancy; Constellation: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Grant/research support ; Celgene: Consultancy; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal